|
Need a New Laboratory Water System?
We have many to choose from. We can save you thousands on
Complete Systems and replacement filters for most brands. |
Water As A Solvent
|
Water is a remarkable substance when used as a cleaning agent sue to its solvent ability. Most substances will dissolve a little bit to completely in many instances. For instance based on water temperature several teaspoons of sucrose or sodium chloride will dissolve in a 250 ml beaker when using deionized water. In fact organic compounds that appear insoluble in water can be found dissolved in trace amounts in most water samples.
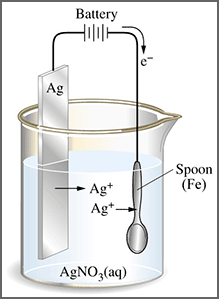
Notably there is a difference in using ultrapure water as a cleanser and as a solvent. In the latter the ultrapure water acts as an agent from bringing together different substances. At least one of these is a solution in order to facilitate a chemical reaction. Unless it is ionized in an aqueous solution sodium chloride and silver nitrate would have no reaction between them.In a solution the form the insoluble precipitate silver chloride. This also forms the soluble ionized sodium nitrate.
One common solvent based process is electroplating. It is the result of the deposition of one substance on another in the form of a thin film. A good example of this would be chromium plating.
 |
In this process the item being plated is immersed in a water solution of sulfuric and chromic acids made up of deionized water with an electrode attached to to the item. As a current goes through the solution the chromium migrates to the the item and becomes deposited on it. Once the process has been running for some time a trivalent chromium contaminant forms. This trivalent chromium contaminant requires a gradual increase in plating current density in order to sustain the rate of deposition. Eventually the current demand reaches a point in which the plating solution must be discarded or purified. To purify this water it must be diluted with deionized water to less than 100 g/l and then pass through a cation exchange chamber. This removes metallic ions. This also leaves pure chromic acid that is ready to be reused. To remove distracting water spots the plated parts are then rinsed in deionized water. |
Even though film is used less and less the solvent and deionizing properties of water are used when processing color film. The chemicals used are prepared in deionized water. As the film development process is done the film is rinsed in deionized water throughout the process and at the end of the process. This results in sharper images and truer colors.
| |
|
Images are representative of the products. Images may or may not be of the actual product. If it is important e-mail us for an actual image if available.
* Flat Rate UPS shipping when able to ship via UPS and is in the USA excluding Hawaii and Alaska.
Larger Items may not be able to ship via UPS, in that case freight charges will be quoted seperately.
International shipping will be quoted after the order is placed. You will have the opportunity to cancel before we finalize your order.
Terms and conditions
Credit Application
Privacy
Policy
List All Products
|